Medical Device & Equipment
Power innovation with confidence—fully managed compliant, secure cloud solutions that keep patient data safe and your innovations ahead.

 Scale smarter.
Scale smarter.
Innovate faster.
Designing life-changing medical devices is complex, but securing data and navigating regulations shouldn’t slow you down.
From integrating device data with healthcare systems to meeting stringent FDA regulations, we’re here to simplify the process with managed cloud compliance, security, and operations services and the CSPM software you can trust, so you stay ready to develop groundbreaking medical technology.
Trust ClearDATA to scale smarter,
and innovate faster.Designing life-changing medical devices is complex, but securing data and navigating regulations shouldn’t slow you down.
We understand the unique challenges you face—from integrating device data with healthcare systems to meeting stringent FDA regulations—and we’re here to simplify the process, so you can focus on delivering groundbreaking medical technology.
Enter our
breach-free zone.

PHI records breached over 14 years in business under ClearDATA management, so you keep sensitive device-generated data secure.
Recover at
lightning speed.

Faster Mean Time to Resolve (MTTR) by our team compared to doing it on your own, so you reduce security risks and ensure uninterrupted device connectivity.
Stay effortlessly
compliant.
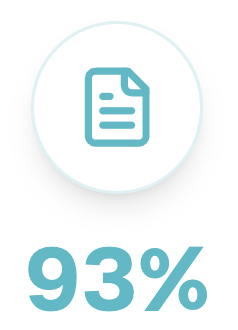
Average continuous compliance score of our customers, ensuring you’ll achieve and maintain FDA, HIPAA, and HITRUST compliance.
Slash costs,
scale faster.

Potential savings in cloud operations costs by working with ClearDATA, so you reduce operational expenses.
Here’s how ClearDATA works for you:
Data interoperability
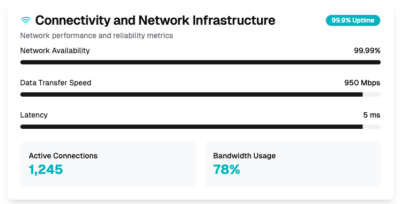
Connectivity infrastructure
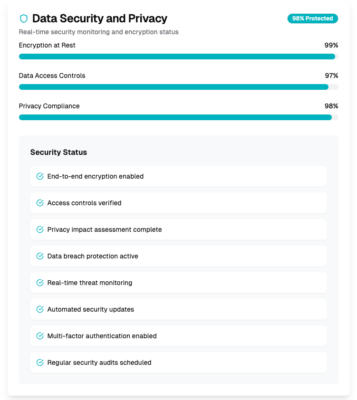
Data security
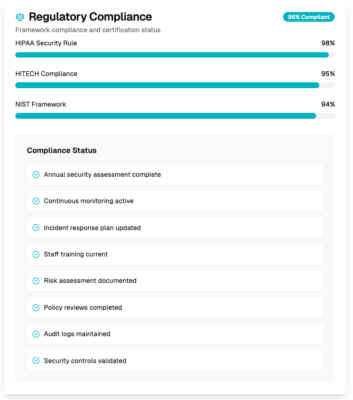
Regulatory compliance
Data Interoperability
Ensure your data interoperability with secure cloud connectivity solutions. We assist in standardizing data formats, implementing secure APIs, and enabling seamless integration with healthcare systems through their professional services.
Credentialed. Celebrated. Certified.