GxP for Life Sciences
Stay ahead of GxP compliance regulations while safeguarding data integrity and security.

Elevate GxP compliance,
build trust.GxP compliance is essential for life sciences organizations that need to maintain regulatory approvals and uphold the quality of healthcare products. We understand the intracies of GxP regulations from agencies like the FDA, EMA, and more.
That’s why we simplify compliance with GxP guidelines – and you maintain the highest standards of data integrity, traceability, and accountability.
Protect sensitive data with encryption and continuous monitoring.
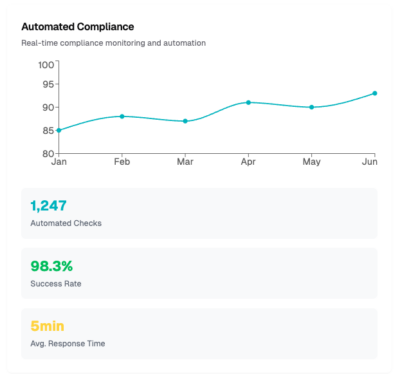
Automate workflows for accurate record-keeping and SOP enforcement.
Ensure consistent manufacturing, testing, and reporting across all stages of production.
We have the expertise and tools to help life sciences organizations operationalize GxP compliance and ensure proactive threat detection and protection.
Work with a partner in innovation that prioritizes quality and patient trust, ensuring your organization is well-prepared for audits, inspections, and evolving regulatory demands.
Here’s how ClearDATA works for you:
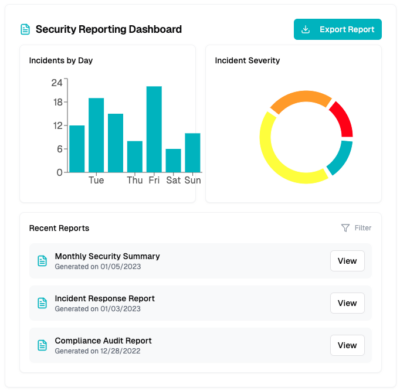
Visibility into security
Operationalize compliance
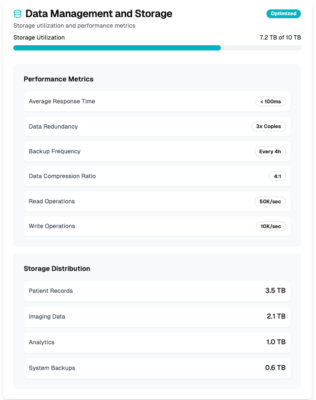
Data management
We enhance security and integration and build visibility
We give you a full view of our dashboard to simplify adherence to security safeguards for meeting GxP standards, providing 24x7x365 security monitoring and protection in AWS, Google, and Azure cloud environments, with PHI/PII discovery capabilities through our compliance and security dashboard in the CyberHealth™ platform, mapped directly to HIPAA, FDA, and GDPR guidelines.
Our professional services team assists you in achieving interoperability, data exchange, and seamless integration between different systems.
We provide client-side and server-side data encryption, encrypted data transfers, data integrity protections, access controls, continuous security monitoring and alerts, and incident management.
Credentialed. Celebrated. Certified.